Observational study
The study has shown that RIFETECH® Plasma is
effective in treating chronic fatigue.
The device uses low-energy electromagnetic frequencies modulated through a plasma emitter. The study included 100 patients and was conducted from April to August 2023. Over a 4-week monitoring period, fatigue was measured using the Fatigue Severity Scale (FSS), while quality of life was assessed through the WHO-Quol-5 questionnaire.
Key findings of the study
Adverse effects were minimal and included (transient) symptom exacerbation, paraesthesia or headache. The study strictly adhered to best clinical practices and was closely monitored.
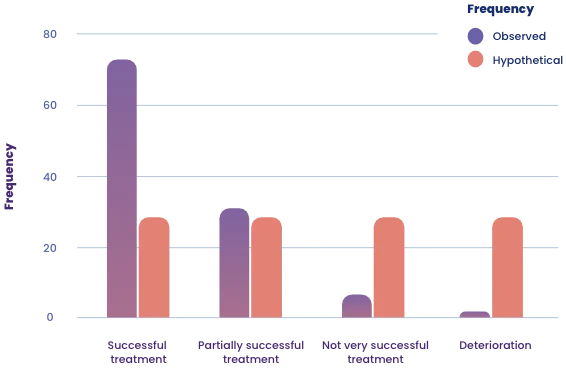
- Following 4 weeks of treatment, both fatigue levels (FSS) and quality of life (WHO-Quol-5) showed statistically significant improvements.
- With a large effect size (Cohen’s d over 0.7), the therapy demonstrated a clinically significant effect.
- The patients treated solely with the RIFETECH® device reported significant improvements.
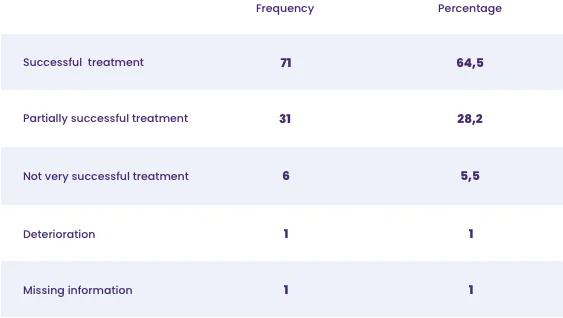
- The medical practitioners described the therapy as a complete success for 64.5 % of the patients, while 28.2 % of the patients were considered to have partially recovered.