The final report on the study led by Professor Harald Walach and Ulrike Kukuk examined the impact of RIFETECH® Plasma, an energy medicine device using plasma-modulated electromagnetic frequencies. The study observed its effect on patients suffering from chronic fatigue. The RIFETECH® Plasma device was used to treat fatigue in 100 patients through 10 therapy sessions over a 4-week period.
Primary objectives and evaluation criteria of the study
The study aimed to evaluate the therapeutic efficacy of the device in patients with fatigue, ICD code G93.3, using the German version of the Fatique Severity Scale (FSS). Additionally, the research explored patients’ quality of life using the WHO-Quol-5 questionnaire. The study, registered as DRKS00031476, included patients presenting diverse levels of exhaustion, treated across 11 practices.
Patients recruitment and study design
A total of 113 patients were enrolled in the study, of whom 110 completed the full course. The patients were recruited from clinics specialising in energy medicine. Patient anonymity was ensured via specific codes, enabling online monitoring and completion of questionnaires. Over the 4-week monitoring period, the patients completed initial and final questionnaires assessing their fatigue levels and quality of life.
Findings
Treatment with RIFETECH® Plasma brought significant improvement to most patients. Fatigue Severity Scale (FSS) scores revealed a significant reduction in fatigue for 110 patients, with statistically significant average improvement (Cohen’s d = 0.87). The patients also reported significant gains in quality of life, as reflected in the WHO-Quol-5 questionnaire (Cohen’s d = 0.76).
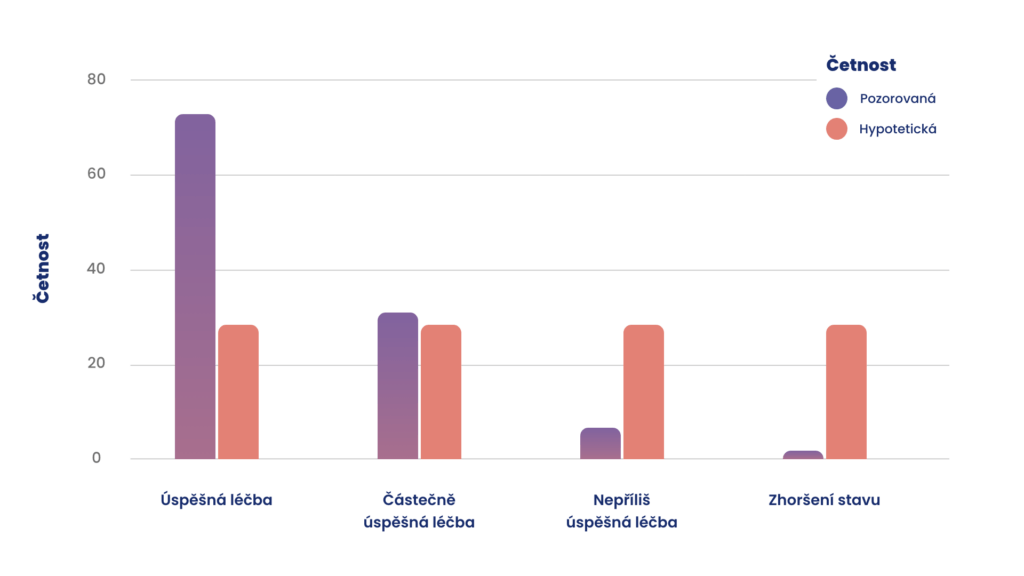
The patients were treated with RIFETECH® Plasma alongside other methods such as dietary supplements, vitamins (especially vitamin D) or naturopathic remedies. The medical practitioners described the therapy as a complete success for 64.5 % of the patients, while 23 % of the patients were considered to have partially recovered.
Adverse effects and other findings
Adverse effects were reported in 23 % of the patients; however, these were predominantly cases of (transient) symptom exacerbation, such as fatigue,paraesthesia or headache. These effects were mild and resolved quickly, posing no serious risks to patients. The medical practitioners also observed additional benefits, such reduced allergy symptoms and reduced fatigue.
Discussion
The findings suggest that RIFETECH® Plasma device treatment significantly benefits chronic fatigue patients. Statistically significant and clinically relevant improvements were observed, even in patients treated exclusively with the device, without any complementary therapies. Overall, the findings confirm that RIFETECH® Plasma makes a significant difference in reducing fatigue.
The report concludes that RIFETECH® Plasma therapy shows promising therapeutic potential for patients suffering from chronic fatigue.
LINK TO THE STUDY: https://drks.de/search/de/trial/DRKS00031476;jsessionid=073919B4654B6A9F9E1611FEA1DB1407
